T. Hatakeyama, S. Ito, M. Nakamura*, E. Nakamura*
J. Am. Chem. Soc. 2005, 127, 14192–14193. DOI: 10.1021/ja055306q
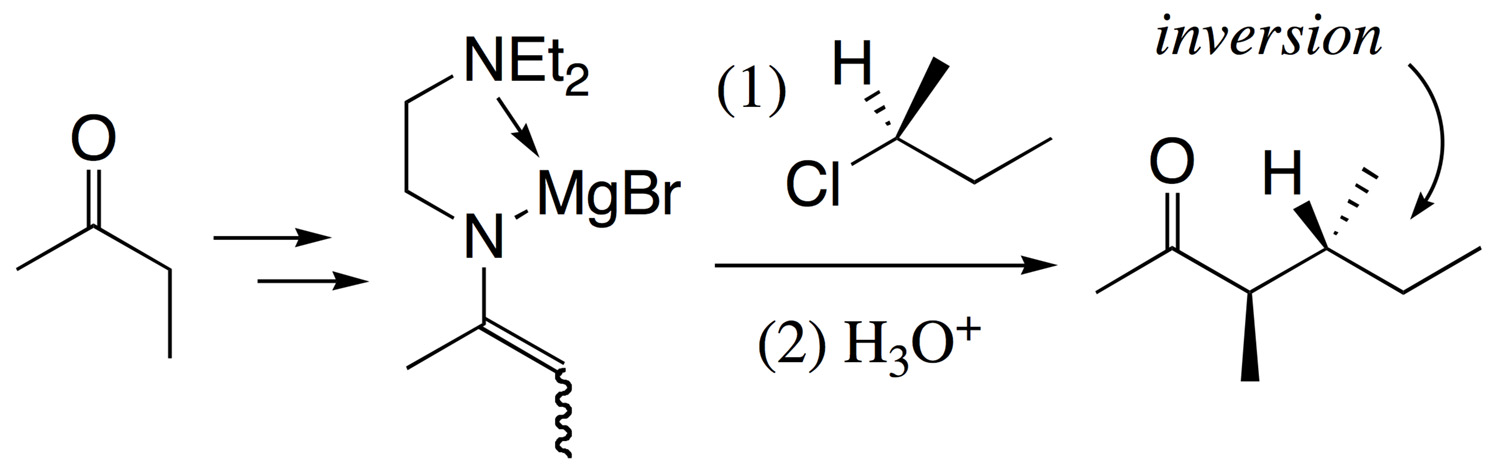
Abstract: A magnesium enamide derived from N-2-(N',N'-diethylamino)ethyl imine reacts with a primary and secondary alkyl chloride or a primary alkyl fluoride to give an α-alkylated ketone in good to excellent yield after hydrolysis. The reaction takes place with a high level of inversion of stereochemistry at the electrophilic carbon center and will be useful for production of optically active compounds.