K. Kanemoto, K. Yoshimura, K. Ono, W. Ding, S. Ito, N. Yoshikai*
Chem. Eur. J. 2024, 30, e202400894. DOI: 10.1002/chem.202400894
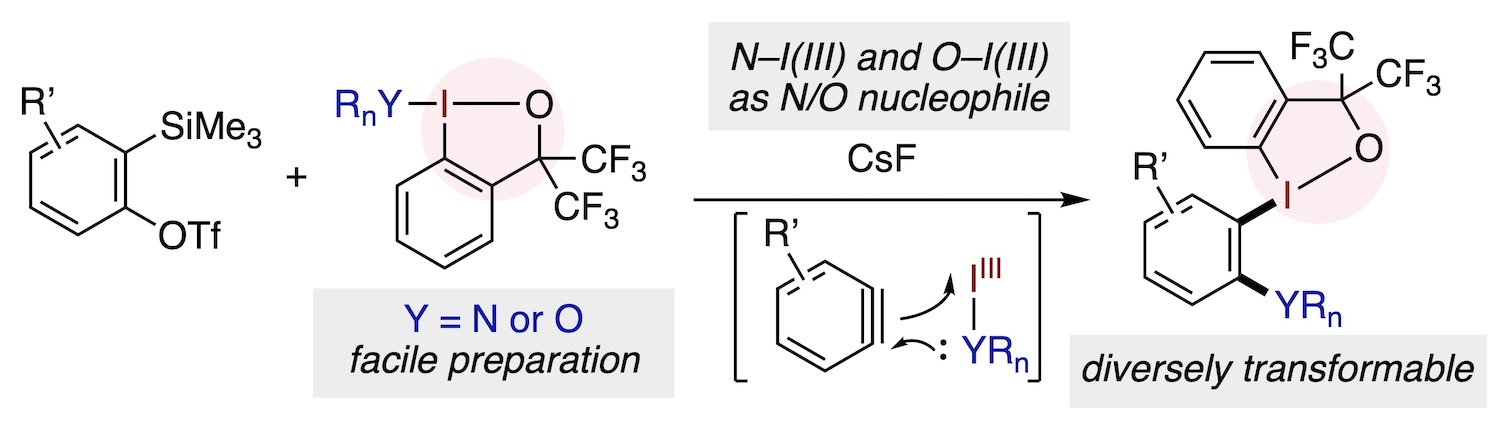
Abstract: We report here on the facile synthesis of amino- and alkoxy-λ3-iodanes supported by a benziodoxole (BX) template and their use as arynophiles. The amino- and alkoxy-BX derivatives can be readily synthesized by reacting the respective amines or alcohols with chlorobenziodoxole in the presence of a suitable base. Unlike previously known nitrogen- and oxygen-bound iodane compounds, which have primarily been employed as electrophilic group transfer agents or oxidants, the present amino- and alkoxy-BX reagents manifest themselves as nucleophilic amino and alkoxy transfer agents toward arynes. This reactivity leads to the aryne insertion into the N–I(III) or O–I(III) bond to afford ortho-amino- and ortho-alkoxy-arylbenziodoxoles, iodane compounds nontrivial to procure by existing methods. The BX group in these insertion products exhibits excellent leaving group ability, enabling diverse downstream transformations.