J. Kikuchi,* T. Nagata, S. Ito, N. Yoshikai*
Org. Chem. Front. 2024, 11, 3072–3079. DOI: 10.1039/D4QO00489B
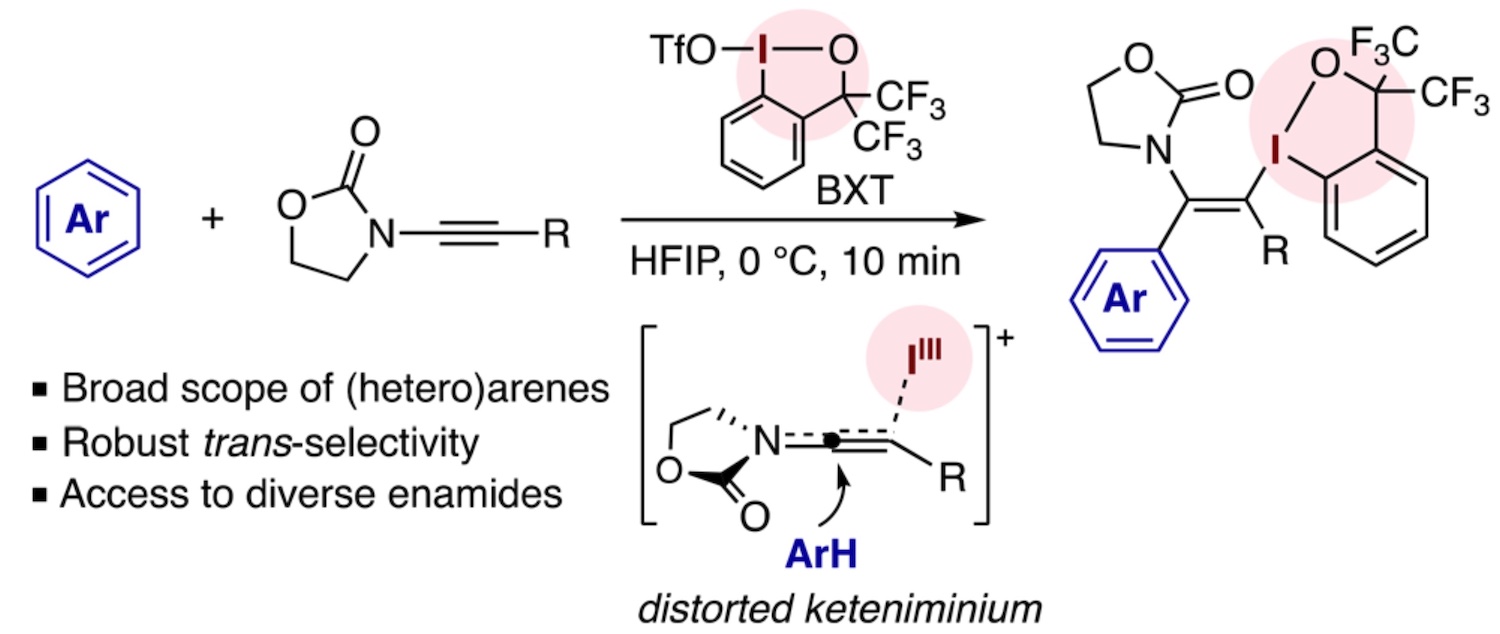
Abstract: The three-component Friedel–Crafts type functionalization of an electron-rich arene using an electrophile-activated alkyne can offer a strategy for rapidly constructing densely functionalized vinylarenes in a regio- and stereoselective fashion. An analogous process utilizing ynamides, a distinct class of electron-rich alkynes, has remained elusive despite its considerable potential for the synthesis of highly substituted and stereodefined enamides. This transformation is particularly challenging with respect to the stereochemical control, as the pivotal intermediate, the electrophile-bound keteniminium ion, can inherently undergo nucleophilic attack from either side of the cumulated double bonds. Here, we report the development of a three-component difunctionalization of ynamides with a cationic iodine(III) electrophile (benziodoxole triflate) and electron-rich (hetero)arenes that consistently displays trans-selectivity irrespective of the steric profiles of the ynamide and the arene. The reaction proceeds quickly under mild conditions across diverse arenes and heteroarenes, producing α-aryl-β-iodanyl enamides in a regio- and stereocontrolled manner. The robust trans-selectivity of this iodo(III)arylation process is attributed to the reactivity of a highly distorted keteniminium species generated from the ynamide and the iodine(III) electrophile. The iodanyl moiety in these reaction products is amenable to a variety of transition metal-mediated coupling, thereby facilitating access to a wide array of densely functionalized enamides.