T. Hatakeyama, S. Ito, H. Yamane, M. Nakamura*, E. Nakamura*
Tetrahedron 2007, 63, 8440–8448. DOI: 10.1016/j.tet.2007.05.087
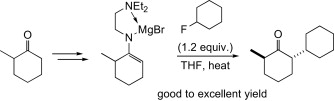
Abstract: A magnesium enamide derived from N-2-(N′,N′-dimethylamino)ethyl imine reacts with primary and secondary alkyl chlorides and fluorides to give an α-alkylated ketone in good to excellent yields upon hydrolysis of the imine moiety. Reactions of the highly nucleophilic magnesium enamide derived from an unsymmetrical ketone take place regioselectively. In addition, the C–C bond formation is stereospecific: a substantial inversion of stereochemistry at the electrophilic carbon center (Walden inversion) was observed, proving its potential utility for the production of optically active compounds.