X. Zhang, D. Li. C. C. H. Tan, F. Hanindita, Y. Hamamoto, A. S. Foster,* S. Kawai,* S. Ito*
Nature Synth. 2024, 3, 1283–1291. DOI: 10.1038/s44160-024-00595-5
This paper was highlighted in Synfacts 2024, 20, 1143. DOI: 10.1055/s-0043-1773595
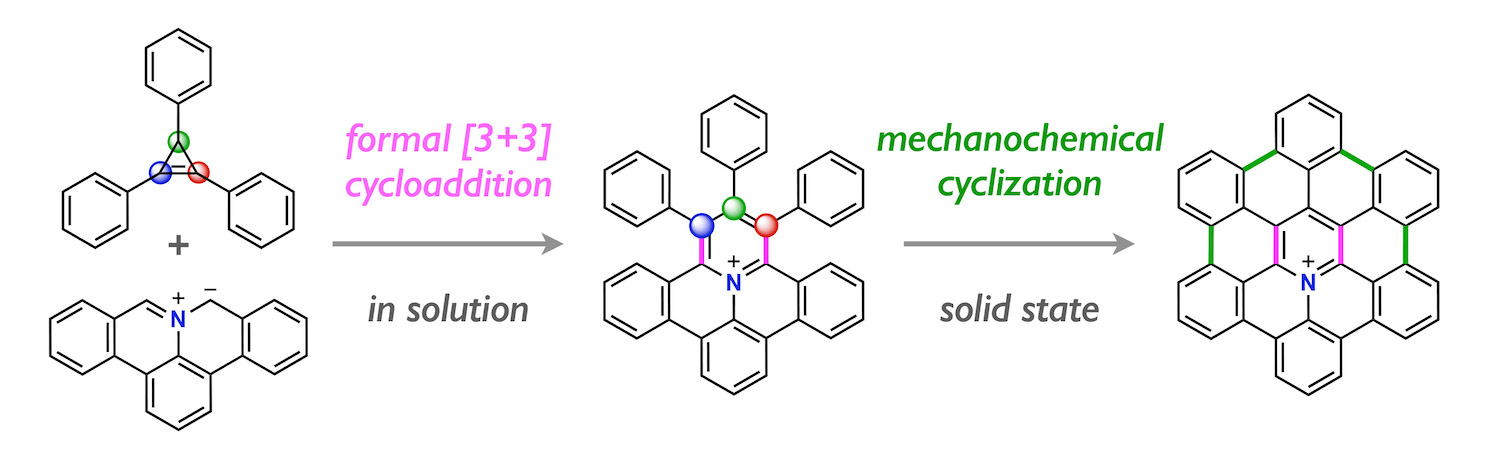
Abstract: Heteroatom-embedded hexa-peri-hexabenzocoronene (HBC) molecules exhibit interesting properties depending on the number and position of the introduced heteroatoms and are promising materials for applications in organic electronics and supramolecular chemistry. However, their synthesis is quite limited because of the difficulty in selectively introducing heteroatoms into the HBC core, which poses a challenge in organic synthesis. Here we report a strategy for the in-solution synthesis of 3a2-azahexa-peri-hexabenzocoronenium salts, which are cationic nitrogen-embedded HBC derivatives. The synthesis was enabled by the formal [3+3] cycloaddition of polycyclic aromatic azomethine ylides with cyclopropenes, as a three-atom dipolarophile, followed by mechanochemical intramolecular cyclization. Furthermore, on-surface polymerization of aza-HBC precursors was performed to synthesize aza-HBC-based chevron-like graphene nanoribbons. This study provides the possibility for the further use of nitrogen-embedded HBC derivatives in a variety of potential applications.