Y. Hamamoto, W. Wang, Y. Li, S. Ito*
Angew. Chem. Int. Ed. 2025, 64, e202416654. DOI: 10.1002/anie.202416654
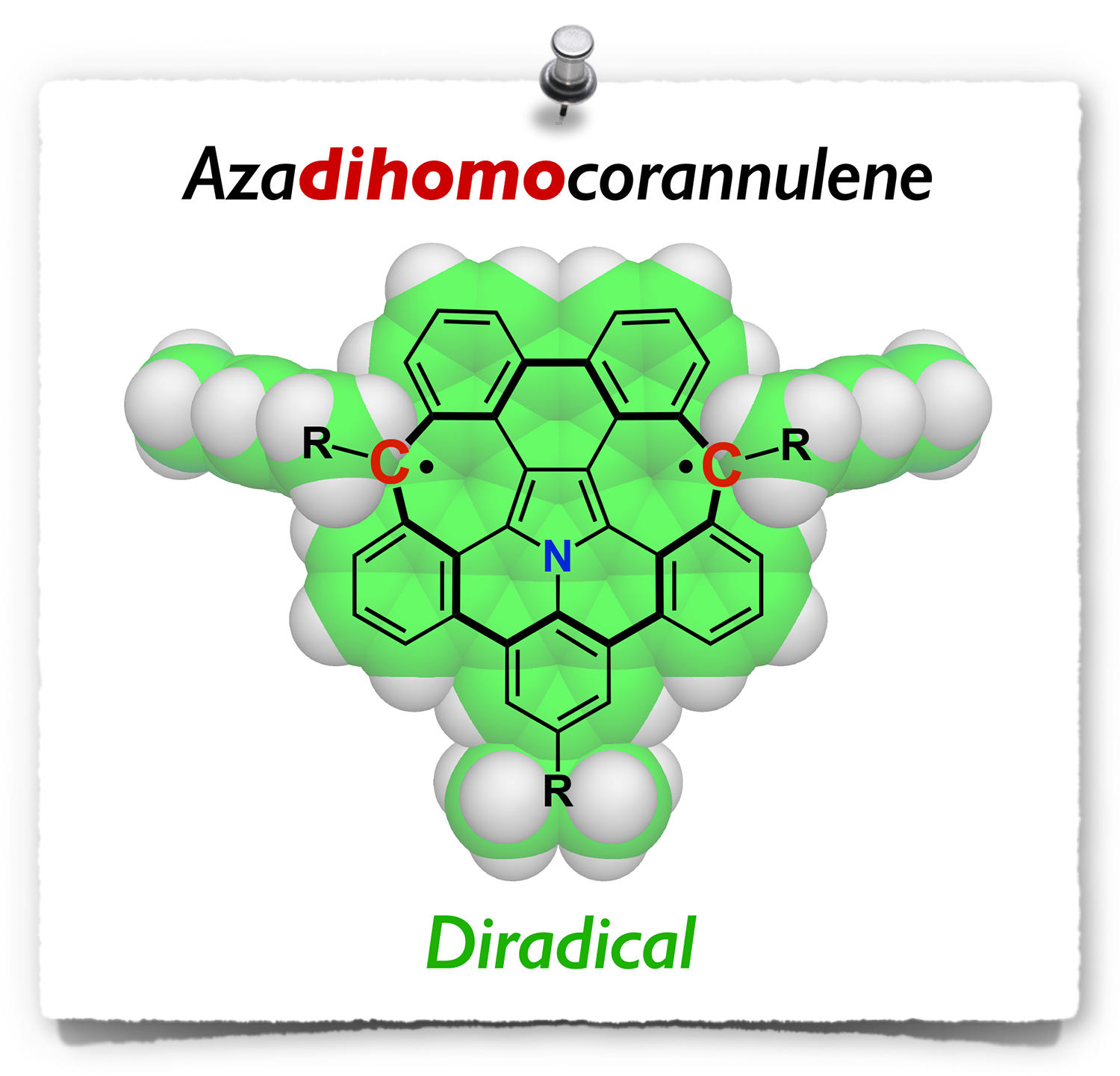
Abstract: Polycyclic aromatic diradical(oid) molecules are attracting significant attention because of their unique electronic and magnetic properties as well as their applications as functional materials. While diradical(oid) molecules bearing five-membered rings have been extensively investigated, those bearing seven-membered rings are relatively fewer. Herein, we report the synthesis of azapentabenzodihomocorannulene dication and diradical molecules. The synthesis was achieved through a mechanochemical C(sp2)–H/C(sp3)–H coupling in the presence of sodium as a key reaction. Electron spin resonance studies revealed that the neutral azapentabenzodihomocorannulene adopts a singlet diradical (diradicaloid) ground state with a small singlet–triplet energy gap of 2.1 kcal/mol. The electronic and optical properties were investigated both experimentally and theoretically to elucidate their aromatic character.