Polycyclic aromatic molecules have attracted much attention because aromatic molecules with reactive π-electrons offer diverse functionality such as conductivity, magnetism, and optical activity, and furthermore acquire advanced functionality upon undergoing higher-order fusion, alignment, or aggregation. Our research is mainly focused on the development of novel synthetic methods for unprecedented polycyclic aromatic molecules and their applications to high-performance organic devices and catalysts.
Synthesis of Heteroatom-Containing π-Functional Molecules
Chemists have been captivated by beautiful shapes of aromatic molecules. One of the goals in our research is to develop novel heteroatom-containing π-functional molecules with unique structures and innovative properties. For example, we have developed polycyclic aromatic azomethine ylides and their 1,3-dipolar cycloaddition reactions with electrophiles, which form a variety of nitrogen-containing planar- and bowl-shaped molecules.
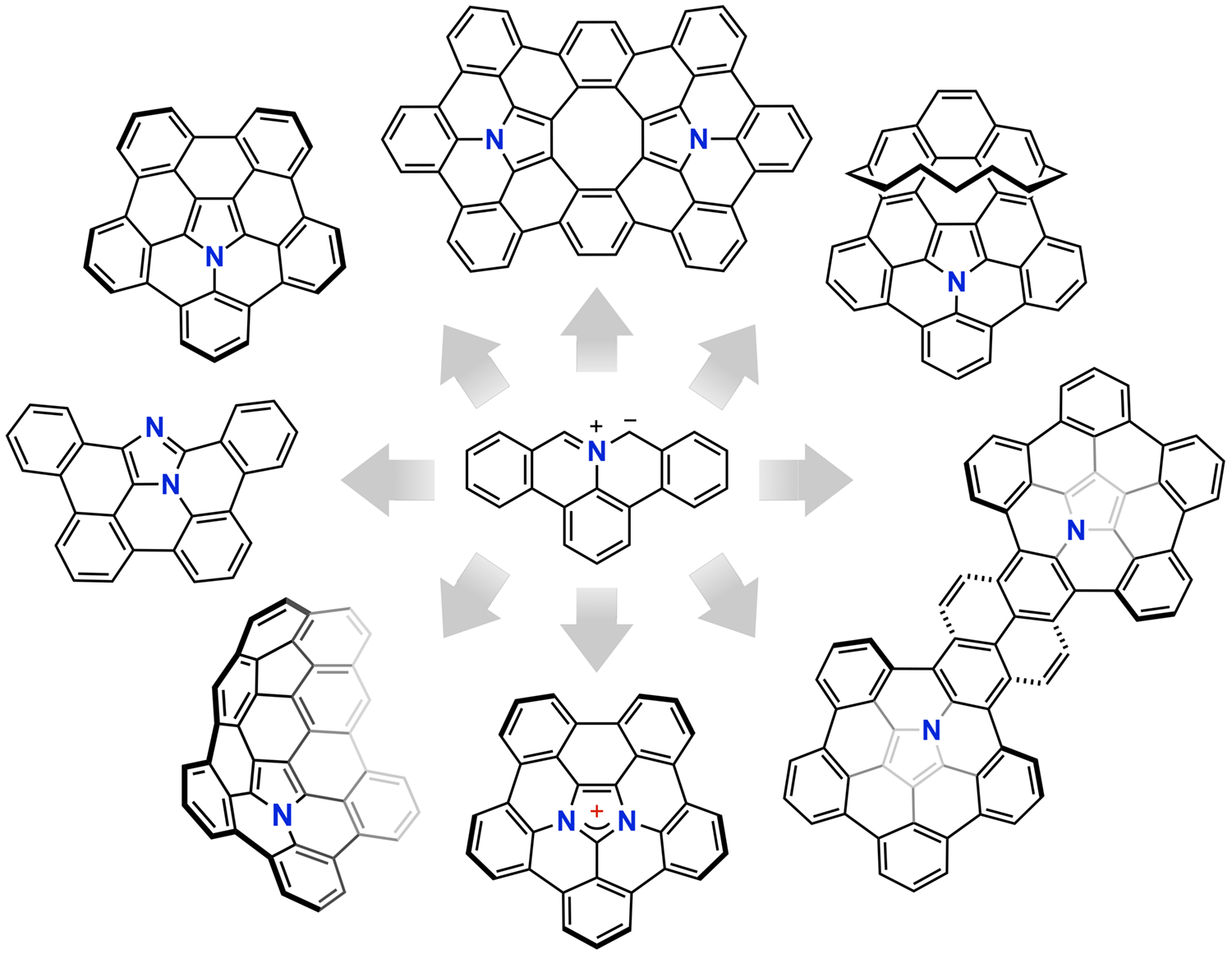
Recent Publication
1) X. Zhang, D. Li. C. C. H. Tan, F. Hanindita, Y. Hamamoto, A. S. Foster, S. Kawai, S. Ito, Nature Synth. 2024, 13, in press. DOI: 10.1038/s44160-024-00595-5
2) Y. Hamamoto, K. Ochiai, Y. Li, E. Tapavicza, S. Ito, Angew. Chem. Int. Ed. 2024, 63, e202319022. DOI: 10.1002/anie.202319022
3) W. Wang, F. Hanindita, Y. Hamamoto, Y. Li, S. Ito, Nat. Commun. 2022, 13, 1498. DOI: 10.1038/s41467-022-29106-w
Development of Novel Synthetic Methodology
We are interested in developing original methods to form novel carbon–carbon bonds for synthesizing aromatic molecules ranging from small molecules to polymeric materials. For example, we have developed “formal aryne polymerization”, which uses oxabicyclic alkenes as aryne equivalents. It is thus possible to access a diverse range of poly(o-arylene)s via a chain-growth mechanism without using unstable arynes.
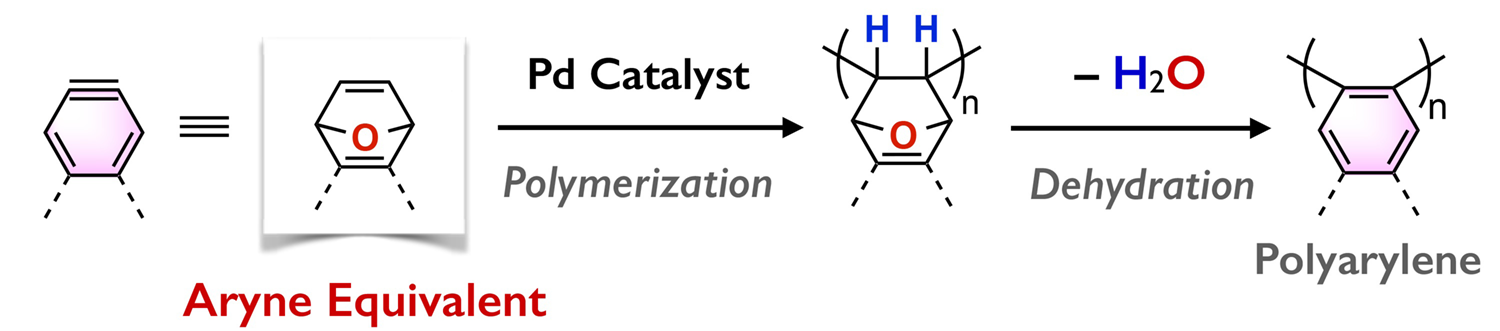
References
1) S. Ito, Bull. Chem. Soc. Jpn. 2018, 91, 251 (Review). DOI: 10.1246/bcsj.20170347
2) S. Ito, Polym. J. 2016, 48, 667 (Review). DOI: 10.1038/pj.2016.18
3) S. Ito, K. Takahashi, K. Nozaki, J. Am. Chem. Soc. 2014, 136, 7547. DOI: 10.1021/ja502073k
