S. Ito*, K. Takahashi, K. Nozaki*
J. Am. Chem. Soc. 2014, 136, 7547–7550. DOI: 10.1021/ja502073k Highlighted in Synfacts.
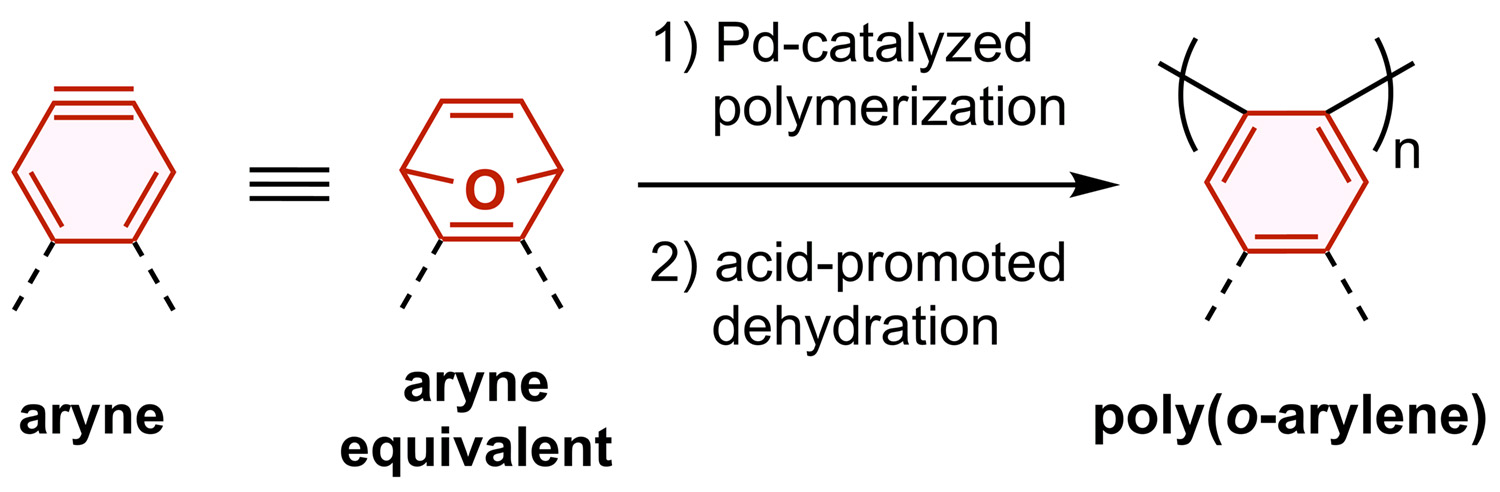
Abstract: Despite the high utility of aryne in organic synthesis, its polymerization has long been a significant challenge in polymer chemistry. A critical bottleneck in this process is the intrinsic instability of aryne and consequent short lifetime for polymerization. In order to circumvent the problem, we focus on a synthetic equivalent of aryne, [2.2.1]oxabicyclic alkene, as a monomer for polymerization. Palladium-catalyzed coordination–insertion polymerization of [2.2.1]oxabicyclic alkenes produced the corresponding polymers having an aliphatic main chain. Subsequent acid-promoted dehydration allowed the aliphatic main chain to be converted into an aromatic main chain to form poly(o-arylene)s. These sequential processes represent the first formal aryne polymerization, which offers an efficient method to synthesize a variety of poly(o-arylene)s in a chain-growth polymerization manner.