S. Ito*, Y. Tokimaru, K. Nozaki*
Angew. Chem. Int. Ed. 2015, 54, 7256–7260. DOI: 10.1002/anie.201502599 Highlighted in Frontispiece and Synfacts
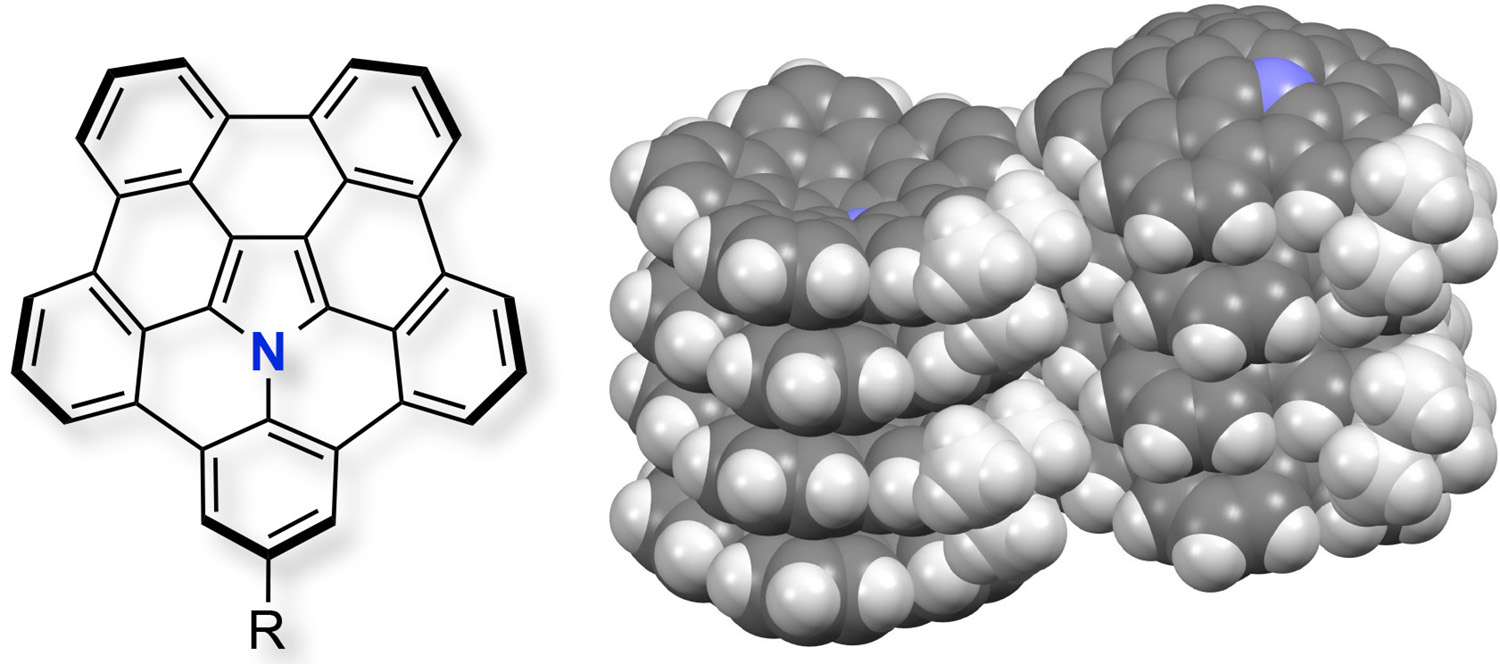
Abstract: A novel benzene-fused azacorannulene was synthesized by the 1,3-dipolar cycloaddition of a polycyclic aromatic azomethine ylide with a diarylethyne and subsequent palladium-catalyzed intramolecular cyclization. This molecule represents the first example of a heteroatom-doped corannulene derivative and in the solid state bowl-in-bowl columnar π-stacking is observed.