Y. Mitsushige, H. Yasuda, B. P. Carrow, S. Ito, M. Kobayashi, T. Tayano, Y. Watanabe, Y. Okuno, S. Hayashi, J. Kuroda, Y. Okumura, K. Nozaki*
ACS Macro Lett. 2018, 7, 305–311. DOI: 10.1021/acsmacrolett.8b00034
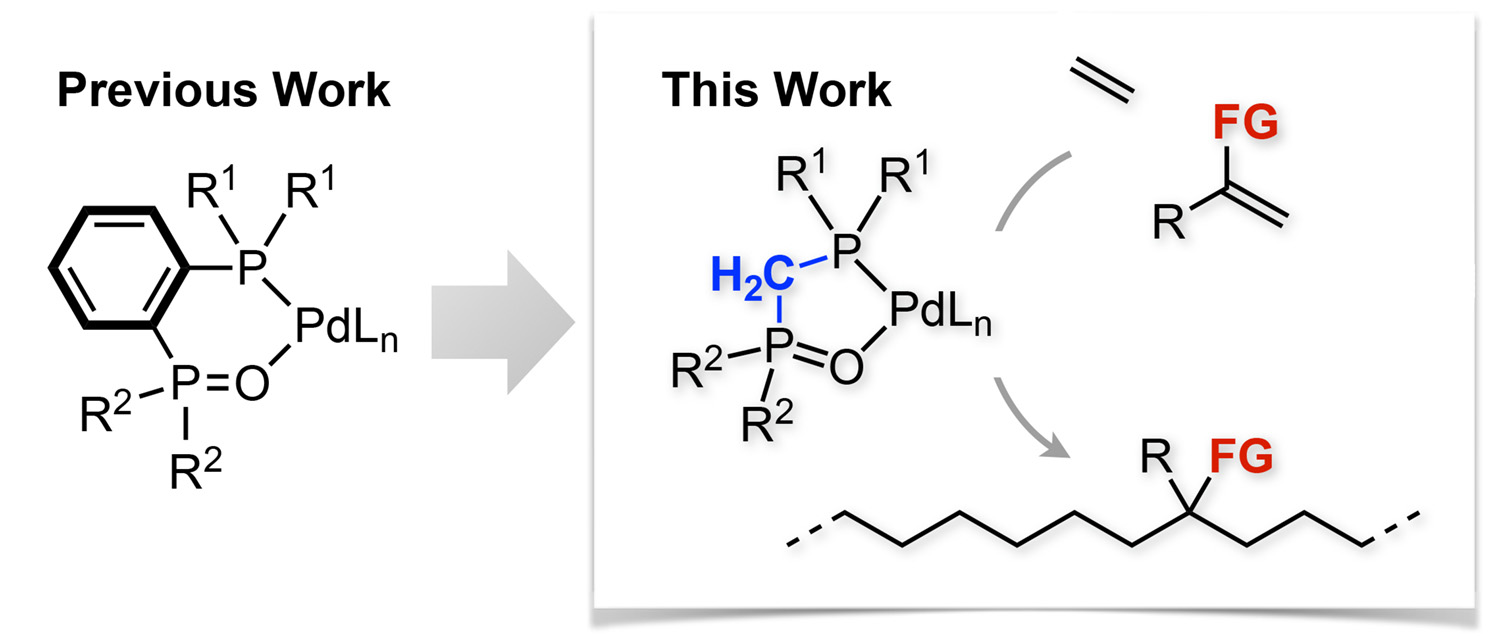
Abstract: A series of palladium complexes bearing a bisphosphine monoxide with a methylene linker, that is, [κ2-P,O-(R12P)CH2P(O)R22]PdMe(2,6-lutidine)][BArF4] (Pd/BPMO), were synthesized and evaluated as catalysts for the homopolymerization of ethylene and the copolymerization of ethylene and polar monomers. X-ray crystallographic analyses revealed that these Pd/BPMO complexes exhibit significantly narrower bite angles and longer Pd−O bonds than Pd/BPMO complexes bearing a phenylene linker, while maintaining almost constant Pd−P bond lengths. Among the complexes synthesized, menthyl-substituted complex 3f (R1 = (1R,2S,5R)-2-isopropyl-5-methylcyclohexan-1-yl; R2 = Me) showed the best catalytic performance in the homo- and copolymerization in terms of molecular weight and polymerization activity. Meanwhile, complex 3e (R1 = t-Bu; R2 = Me) exhibited a markedly higher incorporation of comonomers in the copolymerization of ethylene and allyl acetate (≤12.0 mol %) or methyl methacrylate (≤0.6 mol %). The catalytic system represents one of the first examples of late-transition-metal complexes bearing an alkylene-bridged bidentate ligand that afford high-molecular-weight copolymers from the copolymerization of ethylene and polar monomers.
