Y. Konishi, W. Tao, H. Yasuda, S. Ito, Y. Oishi, H. Ohtaki, A. Tanna, T. Tayano*, K. Nozaki*
ACS Macro Lett. 2018, 7, 213–217. DOI: 10.1021/acsmacrolett.7b00904
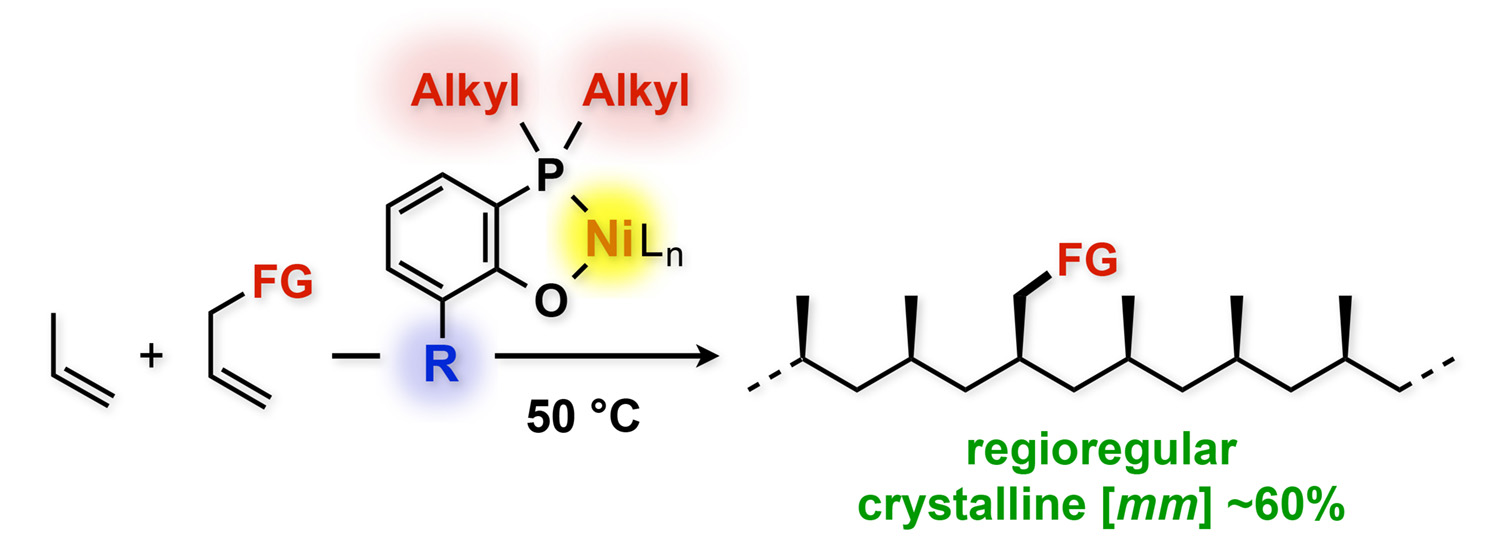
Abstract: Nickel complexes bearing bidentate alkylphosphine–phenolate ligands were synthesized and applied to the polymerization of propylene and the copolymerization of propylene and polar monomers. Therein, the use of bulky alkyl substituents on the phosphorus atom was crucial for the formation of highly regioregular polypropylenes. Theoretical calculations suggested that the 1,2-insertion of propylene is favored over the 2,1-insertion in these nickel-catalyzed (co)polymerization reactions. The substituent at the ortho position relative to the oxido group greatly influences the polymerization activity, the molecular weight, and stereoregularity of the polypropylenes. This method delivers moderately isotactic ([mm] <68%) crystalline polypropylenes. The present system represents the first example for a nickel-catalyzed regio-controlled copolymerization of propylene and polar monomers such as but-3-en-1-ol, but-3-en-1-yl acetate, and tert-butyl allylcarbamate.