Y. Tokimaru, S. Ito*, K. Nozaki*
Angew. Chem. Int. Ed. 2017, 56, 15560–15564. DOI: 10.1002/anie.201707087 Highlighted in Synfacts
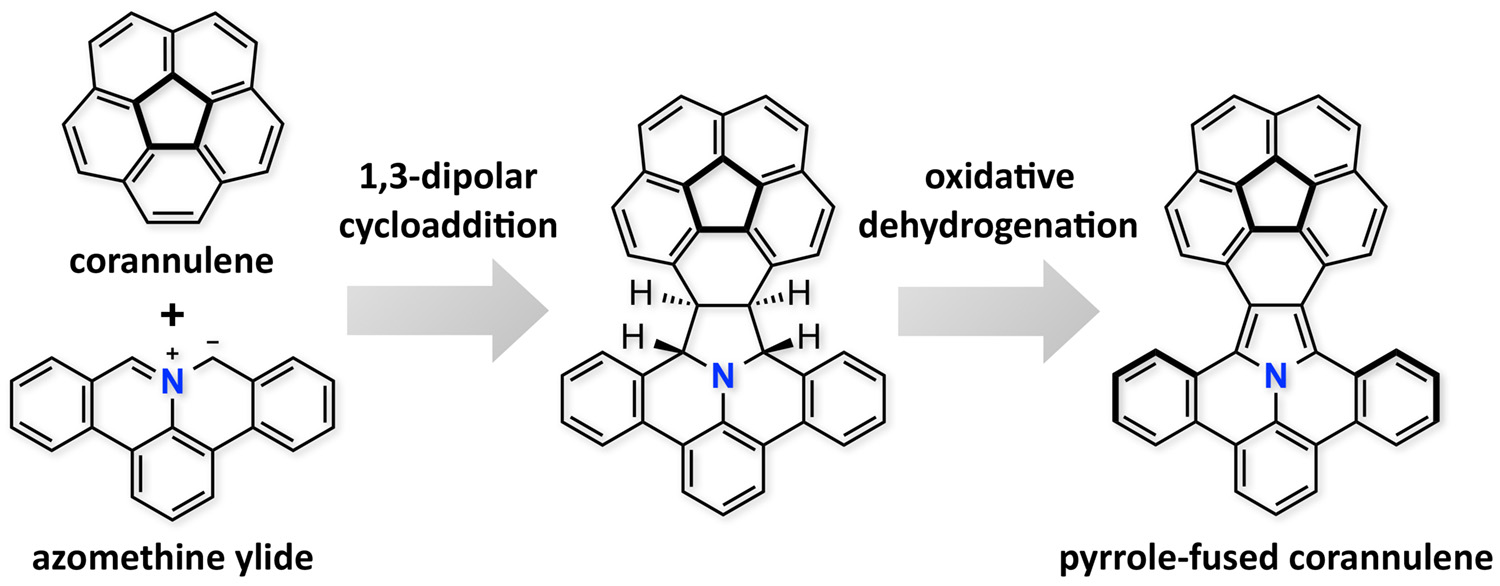
Abstract: In the long history of corannulene chemistry, the 1,3-dipolar cycloaddition to corannulene is unprecedented. Reported herein is the 1,3-dipolar cycloaddition of a polycyclic aromatic azomethine ylide to corannulene, a reaction which occurs exclusively at the rim bond of corannulene, from the convex side in an exo fashion. The cycloadducts were successfully converted, by successive oxidative dehydrogenation, into pyrrole-fused corannulenes, which exhibited pronounced solvatofluorochromism.