S. Kawai*, K. Takahashi, S. Ito*, R. Pawlak, T. Meier, P. Spijker, F. F. Canova, J. Tracey, K. Nozaki, A. S. Foster*, E. Meyer
ACS Nano 2017, 11, 8122–8130. DOI: 10.1021/acsnano.7b02973
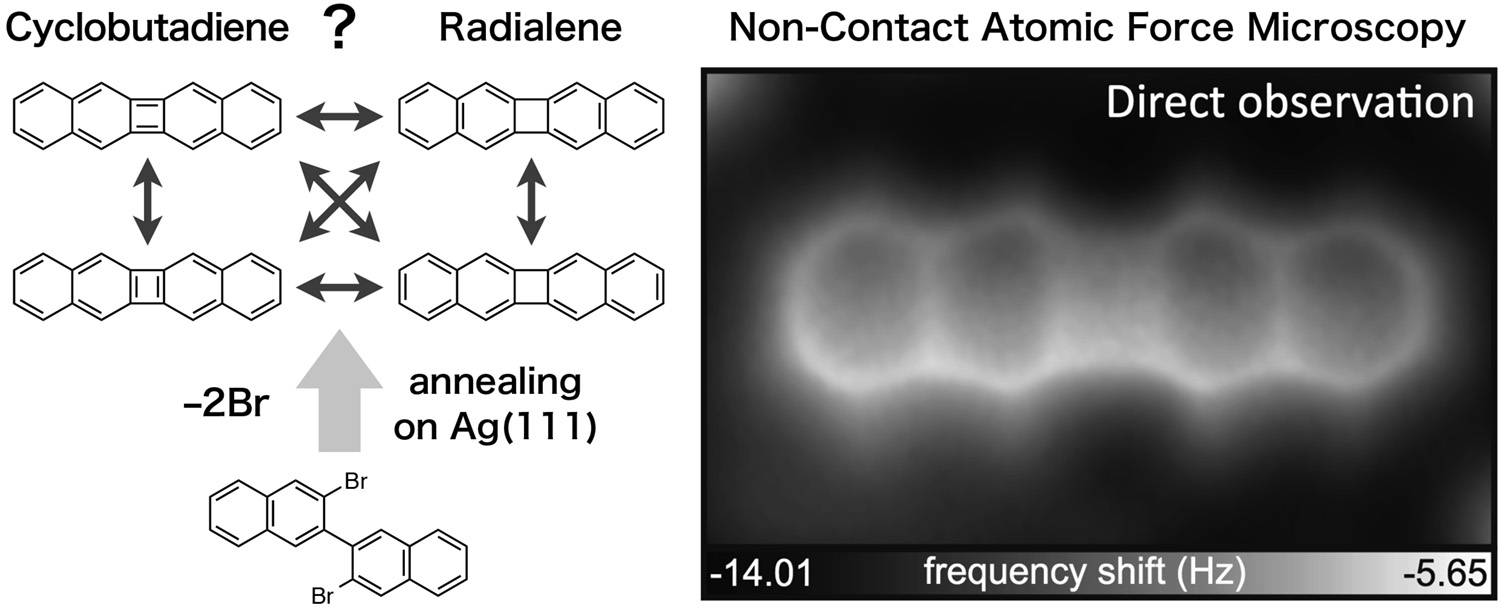
Abstract: According to Hückel theory, an anti-aromatic molecule possessing (4n)π-electrons becomes unstable. Although the stabilization has been demonstrated by radialene-type structures—fusing aromatic rings to anti-aromatic rings—in solution, such molecules have never been studied at a single molecular level. Here, we synthesize a cyclobutadiene derivative, dibenzo[b,h]biphenylene, by an on-surface intramolecular reaction. With a combination of high-resolution atomic force microscopy and density functional theory calculations, we found that a radialene structure significantly reduces the anti-aromaticity of the cyclobutadiene core, extracting π-electrons, while the small four-membered cyclic structure keeps a high density of the total charge.