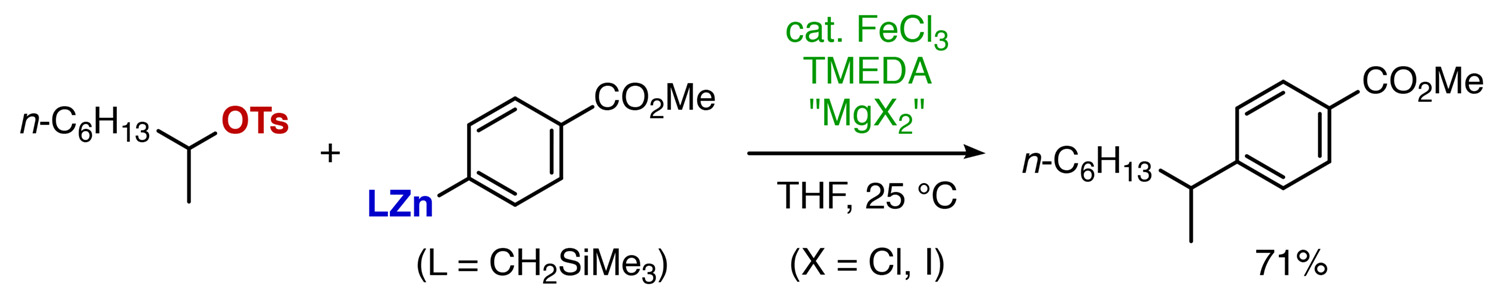S. Ito, Y. Fujiwara, E. Nakamura, M. Nakamura*
Org. Lett. 2009, 11, 4306–4309. DOI: 10.1021/ol901610a
Abstract: Iron-catalyzed cross-coupling reactions of primary and secondary alkyl sulfonates with arylzinc reagents proceed smoothly in the presence of excess TMEDA and a concomitant magnesium salt. The arylzinc reagents are prepared from the corresponding aryllithium or magnesium reagents with ZnI2. The in situ formation of alkyl iodides and consecutive rapid cross-coupling avoids discrete preparation of the unstable secondary alkyl halides and also achieves high product selectivity.