W. Wang, F. Hanindita, Y. Hamamoto, Y. Li, S. Ito*
Nat. Commun. 2022, 13, 1498. DOI: 10.1038/s41467-022-29106-w
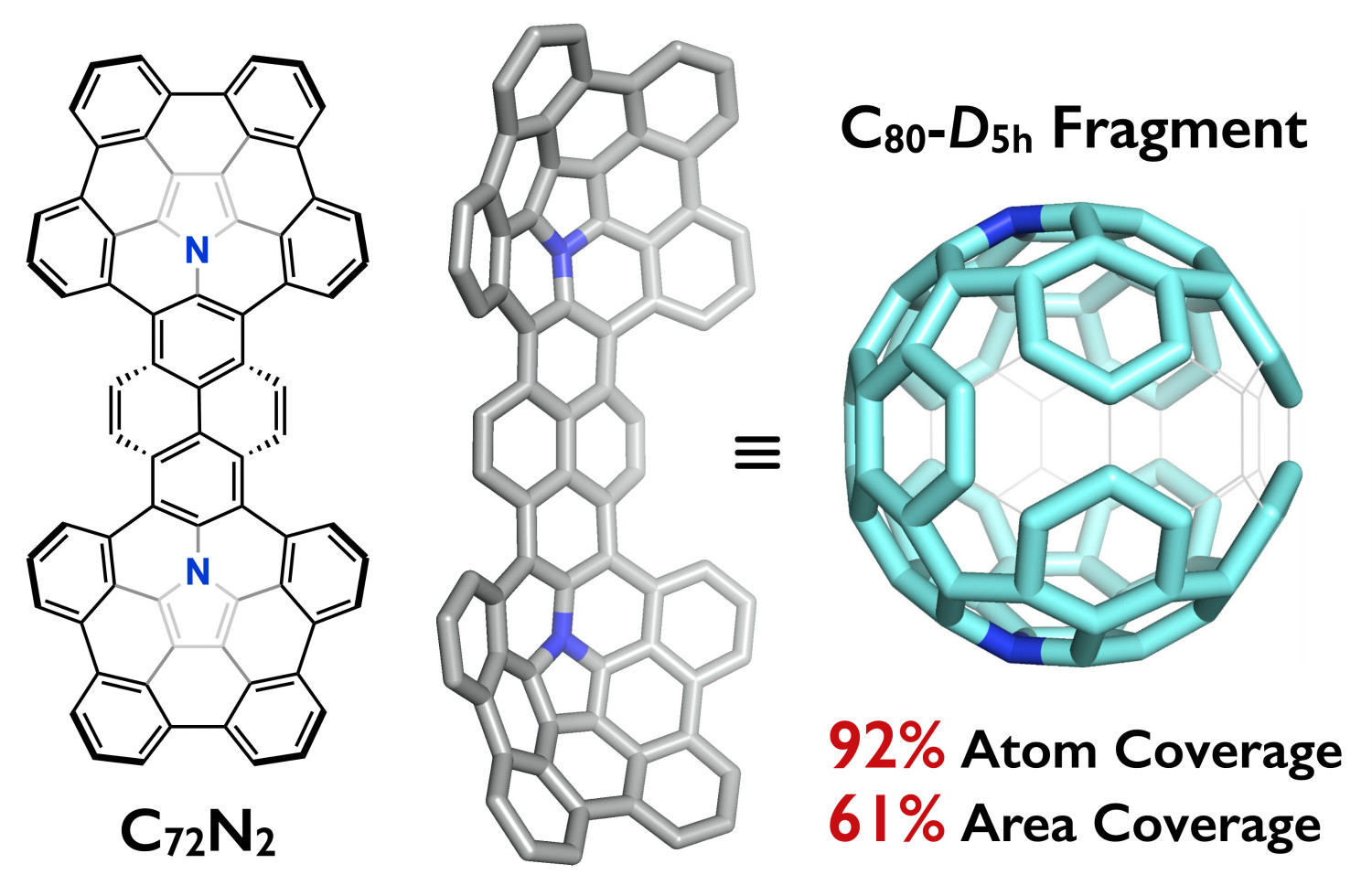
Abstract: A fully conjugated azacorannulene dimer with a large π-surface (76π system) was successfully synthesized from a fully conjugated bifunctional polycyclic aromatic azomethine ylide. This molecule represents an example of diaza[80]fullerene (C78N2) fragment molecule bearing two internal nitrogen atoms. X-ray crystallography analysis shows its boat-shaped structure with two terminal azacorannulenes bent in the same direction. The molecular shape leads to unique selective association with a dumbbell-shaped C60 dimer (C120) over C60 through shape recognition. Owing to its large π-surface and a narrow HOMO–LUMO gap, the azacorannulene dimer exhibits red fluorescence with a quantum yield of up to 31%. The utilization of the fully conjugated bifunctional azomethine ylide is a powerful method for the bottom-up synthesis of large multiazafullerene fragments, providing a step towards the selective total synthesis of multiazafullerenes.