X. Zhang, M. R. Mackinnon, G. J. Bodwell,* S. Ito*
Angew. Chem. Int. Ed. 2022, 61, e202116585. DOI: 10.1002/anie.202116585
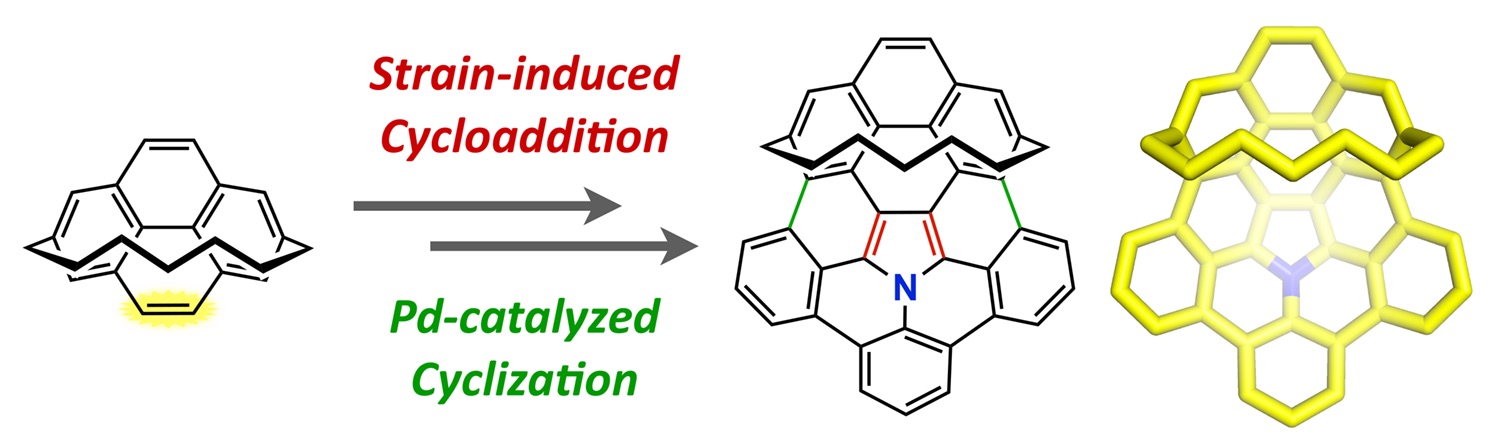
Abstract: The first example of a cyclophane bearing a nitrogen-containing buckybowl was synthesized via sequential 1,3-dipolar cycloaddition and palladium-catalyzed intramolecular cyclization. The key to the successful synthesis is the strain-induced 1,3-dipolar cycloaddition of a polycyclic aromatic azomethine ylide to the K-region of [7](2,7)pyrenophane. The resulting π-extended azacorannulenophane exhibits intriguing structural and physical properties, including unique variation of bowl depth, extraordinarily high-field chemical shifts in its 1H NMR spectrum, a decreased HOMO–LUMO gap, and a red shift in the absorption/emission spectrum, when compared to those of the parent azacorannulene. These characteristics are derived from both the π-extension to the polycyclic aromatic system in the cyclophane structure and the increased curvature enforced by the seven-carbon aliphatic chain.