J. Chai, W. Ding, C. Wang, S. Ito, J. Wu,* N. Yoshikai*
Chem. Sci. 2021, 12, 15128-15133. DOI: 10.1039/D1SC05240C
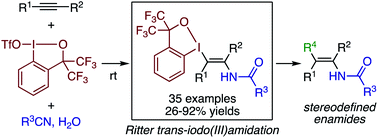
Abstract: The Ritter reaction, Brønsted- or Lewis acid-mediated amidation of alkene or alcohol with nitrile via a carbocation, represents a classical method for the synthesis of tertiary amides. Although analogous reaction through a vinyl cation or a species alike may offer a route to enamide, an important synthetic building block as well as a common functionality in bioactive compounds, such transformations remain largely elusive. Herein, we report on a Ritter-type trans-difunctionalization of alkynes with trivalent iodine electrophile and nitrile, which affords β-iodanyl enamides in moderate to good yields. Mediated by benziodoxole triflate (BXT), the reaction proves applicable to a variety of internal alkynes as well as to various alkyl- and arylnitriles. The benziodoxole group in the product serves as a versatile handle for further transformations, thus allowing for the preparation of various tri- and tetrasubstituted enamides that are not readily accessible by other means.