Y. Mitsushige, B. P. Carrow, S. Ito, K. Nozaki*
Chem. Sci. 2016, 7, 737–744. DOI: 10.1039/C5SC03361F
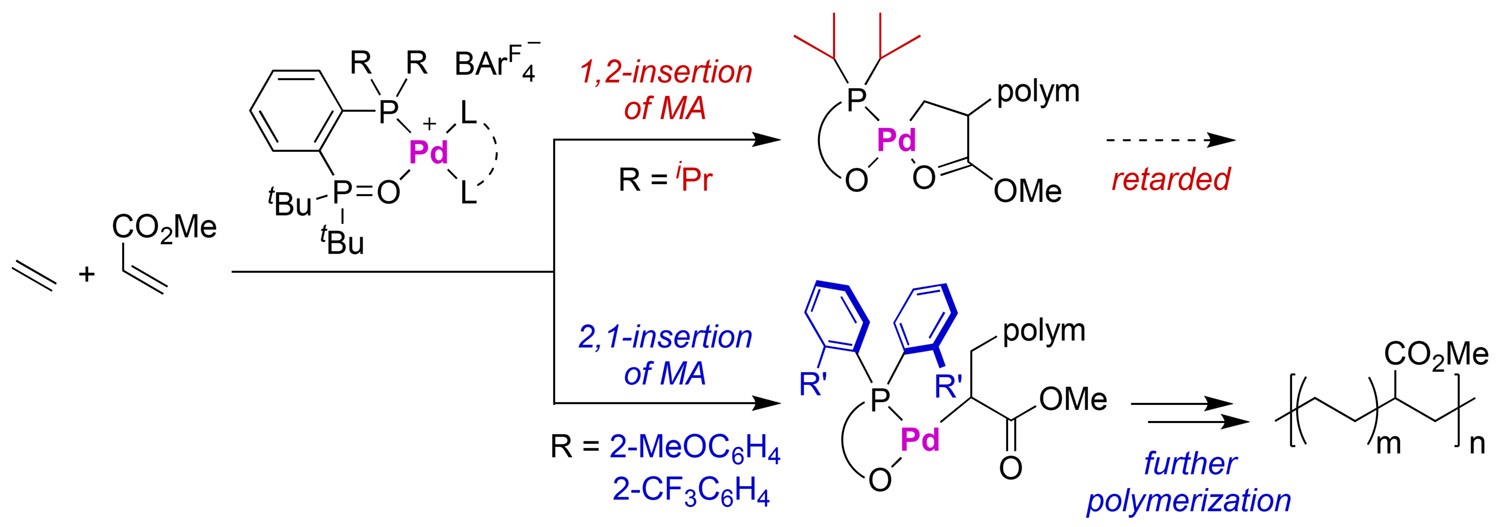
Abstract: A new series of palladium catalysts ligated by a chelating bisphosphine monoxide bearing diarylphosphino groups (aryl-BPMO) exhibits markedly higher reactivity for ethylene/methyl acrylate copolymerisation when compared to the first generation of alkyl-BPMO–palladium catalysts that contain a dialkylphosphino moiety. Mechanistic studies suggest that the origin of this disparate catalyst behavior is a change in regioselectivity of migratory insertion of the acrylate comonomer as a function of the phosphine substituents. The best aryl-BPMO–palladium catalysts for these copolymerisations were shown to undergo exclusively 2,1-insertion, and this high regioselectivity avoids formation of a poorly reactive palladacycle intermediate. Furthermore, the aryl-BPMO–palladium catalysts can copolymerise ethylene with other industrially important polar monomers.