Y. Ota, S. Ito, M. Kobayashi, S. Kitade, K. Sakata, T. Tayano, K. Nozaki*
Angew. Chem. Int. Ed. 2016, 55, 7505–7509. DOI: 10.1002/anie.201600819
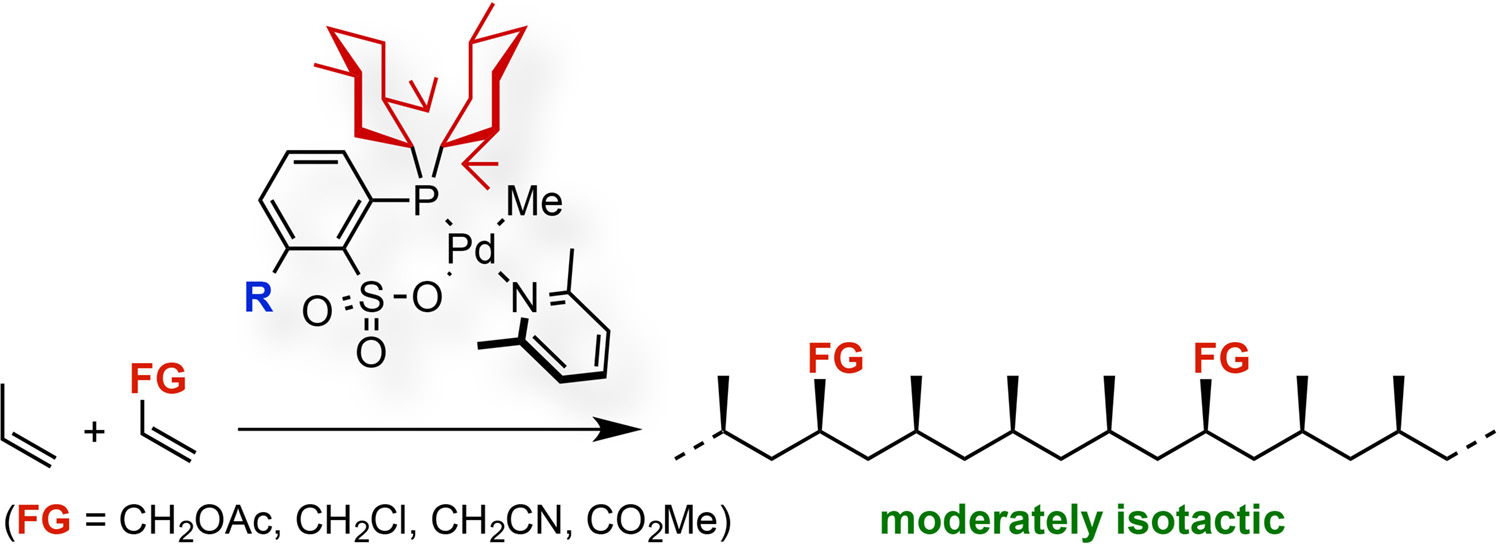
Abstract: Moderately isospecific homopolymerization of propylene and the copolymerization of propylene and polar monomers have been achieved with palladium complexes bearing a phosphine-sulfonate ligand. Optimization of substituents on the phosphorus atom of the ligand revealed that the presence of bulky alkyl groups (e.g. menthyl) is crucial for the generation of high-molecular-weight polypropylenes (Mw ≈ 104), and the substituent at the ortho-position relative to the sulfonate group influences the molecular weight and isotactic regularity of the obtained polypropylenes. Statistical analysis suggested that the introduction of substituents at the ortho-position relative to the sulfonate group favors enantiomorphic site control over chain end control in the chain propagation step. The triad isotacticity could be increased to mm = 0.55–0.59, with formation of crystalline polar polypropylenes, as supported by the presence of melting points and sharp peaks in the corresponding X-ray diffraction patterns.