C.-S. Wang, P. S. L. Tan, W. Ding, S. Ito, N. Yoshikai*
Org. Lett. 2022, 24, 430-434. DOI: 10.1021/acs.orglett.1c04123
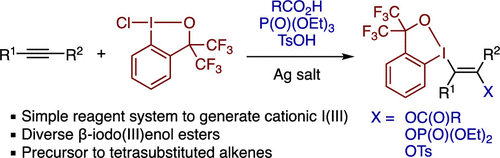
Abstract: β-Iodo(III)enol carboxylates, phosphates, and tosylates can be efficiently synthesized through regio- and stereoselective iodo(III)functionalization of alkynes. The combination of chlorobenziodoxole and silver salt has proven to generate a versatile cationic iodine(III) electrophile to activate alkynes and engage various carboxylic acids, triethyl phosphate, and p-toluenesulfonic acid as nucleophiles. The β-iodo(III)enol esters serve as starting materials for the synthesis of multisubstituted alkenes through sequential cross-coupling of the C–I(III) and C–O bonds.