C. Arakawa, K. Kanemoto, K. Nakai, C. Wang, S. Morohashi, E. Kwon, S. Ito, N. Yoshikai*
J. Am. Chem. Soc. 2024, 146, 3910–3919. DOI: 10.1021/jacs.3c11524
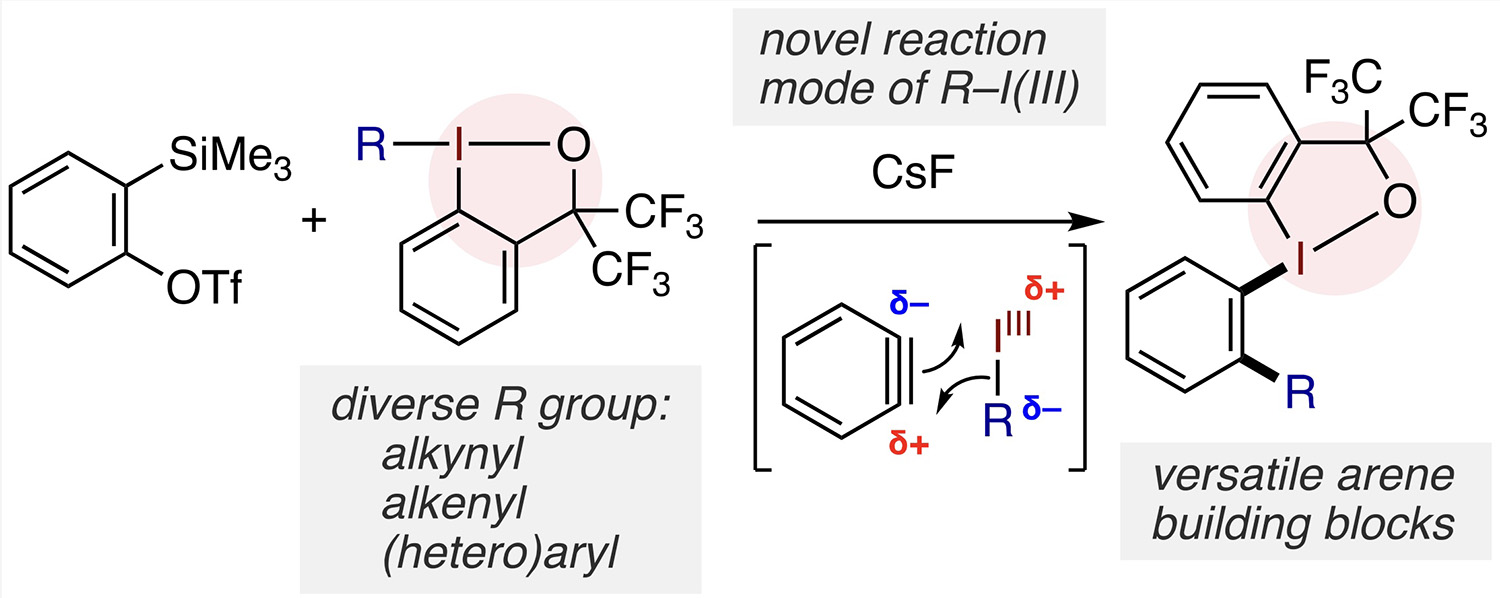
Abstract: Organic iodine(III) compounds represent the most widely used hypervalent halogen compounds in organic synthesis, where they typically perform the role of an electrophile or oxidant to functionalize electron-rich or nucleophilic organic compounds. In contrast to this convention, we discovered their unique reactivity as organometallic-like nucleophiles toward arynes. Equipped with diverse transferable ligands and supported by a tethered spectator ligand, the organoiodine(III) compounds undergo addition across the electrophilic C–C triple bond of arynes while retaining the trivalency of the iodine center. This carboiodanation reaction can forge a variety of aryl–alkynyl, aryl–alkenyl, and aryl–(hetero)aryl bonds along with the concurrent formation of an aryl–iodine(III) bond under mild conditions. The newly formed aryl–iodine(III) bond serves as a versatile linchpin for downstream transformations, particularly as an electrophilic reaction site. The amphoteric nature of the iodine(III) group as a metalloid and a leaving group in this sequence enables the flexible and expedient synthesis of extended π-conjugated molecules and privileged biarylphosphine ligands, where all the iodine(III)-containing compounds can be handled as air- and thermally stable materials.