J. Kikuchi, K. Maesaki, S. Sasaki, W. Wang, S. Ito, N. Yoshikai*
Org. Lett. 2022, 24, 6914–6918. DOI: 10.1021/acs.orglett.2c02570
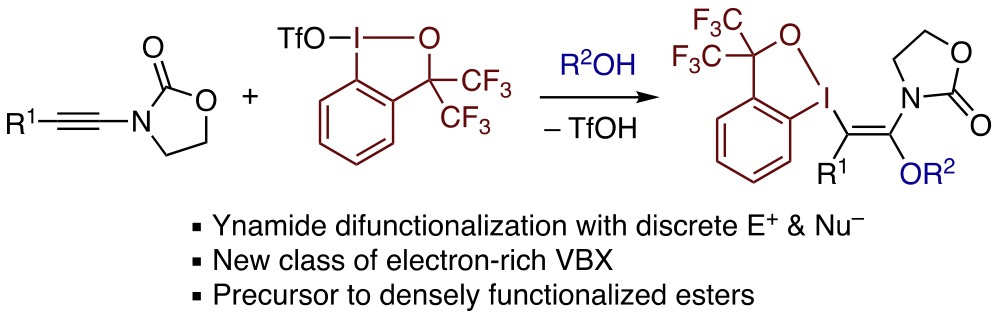
Abstract: A trans-iodo(III)etherification reaction of ynamides with benziodoxole triflate and alcohols is reported. Despite the sensitivity of ynamides and enamides toward Brønsted acid, the reaction could be successfully performed under carefully controlled conditions to afford β-alkoxy-β-amido vinylbenziodoxoles in moderate to good yields. The products could be subjected to a sequence of cross-coupling via C–I(III) bond cleavage and electrophilic halogenation of the resulting α-alkoxyenamides, allowing for the preparation of densely functionalized esters.