Q.-Q. Li, K. Ochiai, C.-A. Lee, S. Ito*
Org. Lett. 2020, 22, 6132–6137. DOI: 10.1021/acs.orglett.0c02203
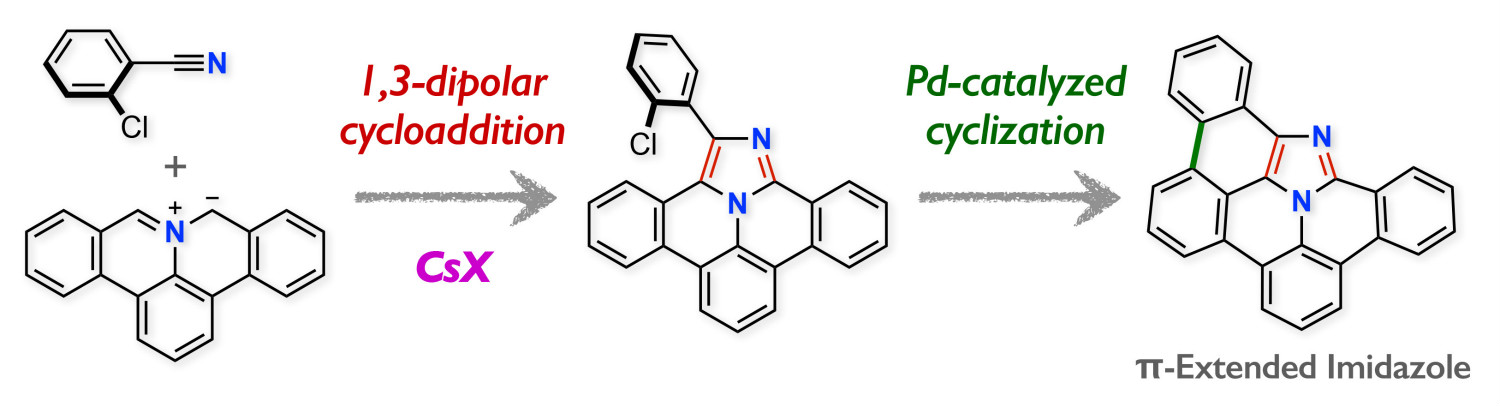
Abstract: Here we report the 1,3-dipolar cycloaddition of polycyclic aromatic azomethine ylides with nitriles to produce highly fused imidazole derivatives, that is, tribenzo[b,g,ij]imidazo[2,1,5-de]quinolizine. The advantages of this transformation are the broad substrate scope and the good functional group compatibility. The subsequent palladium-catalyzed intramolecular cyclization provides an efficient approach to further π-extended imidazoles, that is, 14b1,15-diazadibenzo[fg,ij]cyclopenta[rst]pentaphene.