T. Nagano, K. Nakamura, Y. Tokimaru, S. Ito,* D. Miyajima, T. Aida, K. Nozaki*
Chem. Eur. J. 2018, 24, 14075–14078. DOI: 10.1002/chem.201803676
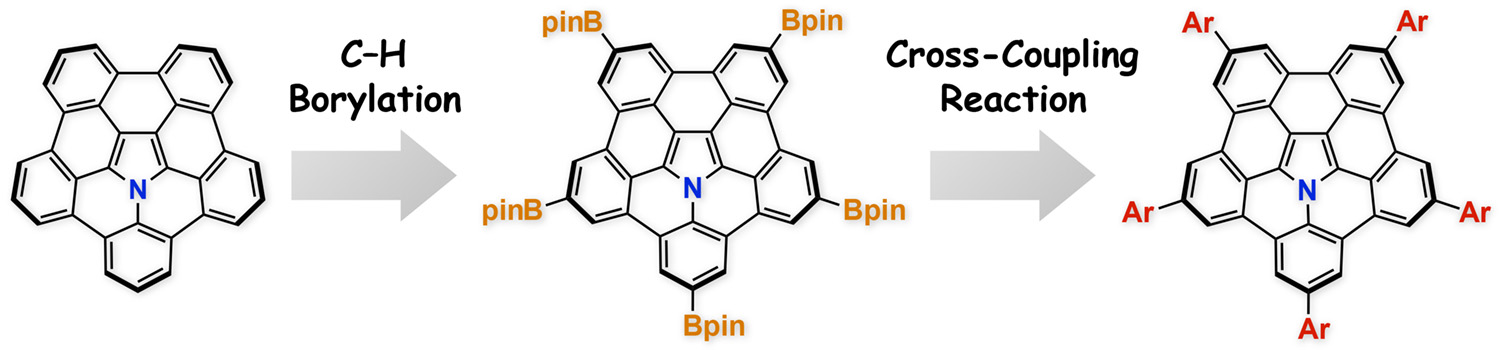
Abstract: Herein, we report the one-shot fivefold functionalization of azapentabenzocorannulenes by an iridium-catalyzed five-fold C–H borylation reaction that exhibits excellent regioselectivity. The borylated product can be used as a versatile synthetic intermediate for further derivatization via Suzuki–Miyaura cross-coupling reactions. This fivefold borylation/arylation sequence was employed to synthesize liquid-crystalline azapentabenzocorannulenes with five 3,4,5-trialkoxyphenyl groups, which assemble into 1D hexagonal columnar structures over a wide temperature range. The present method expands the variety and utility of azapentabenzocorannulenes as promising π-conjugated cores.
