W. Wang, F. Hanindita, R. D. Webster, S. Ito*
CCS Chem. 2023, 5, 1108-1117. DOI: 10.31635/ccschem.022.202202165
Prof. Ito was featured in Auther Spotlight
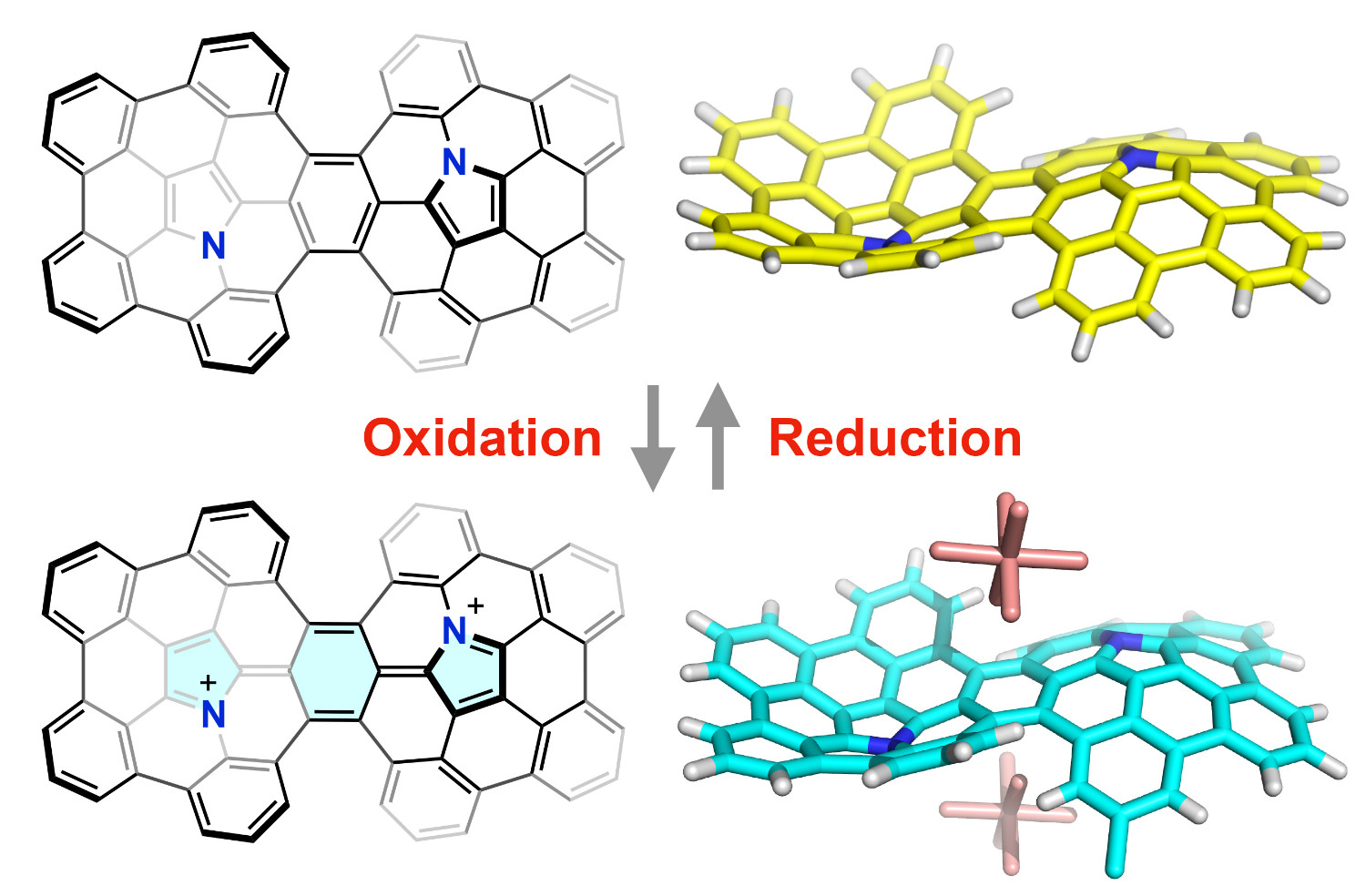
Abstract: Because of their unique structures and properties, buckybowl molecules have attracted considerable attention in a wide range of scientific disciplines. The importance and utility of buckybowl molecules will significantly increase once they acquire larger π-surface area and/or heteroatoms. The fusion of buckybowl molecules has emerged as a new strategy to extend the π-surface of polycyclic aromatic compounds, but the π-extension of heteroatom-embedded buckybowls by the fusion strategy is still rare. Here we report the synthesis and propeties of a fused azacorannulene dimer bearing a C62N2 core (1a), which can also be regarded as a double aza[5]helicene. Due to the steric repulsion between two azapentabenzocorannulene moieties, this molecule shows a rigid S-shaped structure where the two azacorannulene bowls face in an opposite direction. Stepwise chemical oxidation of 1a resulted in the formation of the corresponding radical cation (1a·+) and dication (1a2+), providing an important insight into their aromaticity. The fusion of heteroatom-embedded buckybowls provides a powerful way to synthesize π-extended polycyclic aromatic molecules.