Y. Hamamoto, K. Ochiai, Y. Li, E. Tapavicza, S. Ito*
Angew. Chem. Int. Ed. 2024, 63, e202319022. DOI: 10.1002/anie.202319022
This paper was highlighted in Synfacts 2024, 20, 0473. DOI: 10.1055/s-0043-1763887
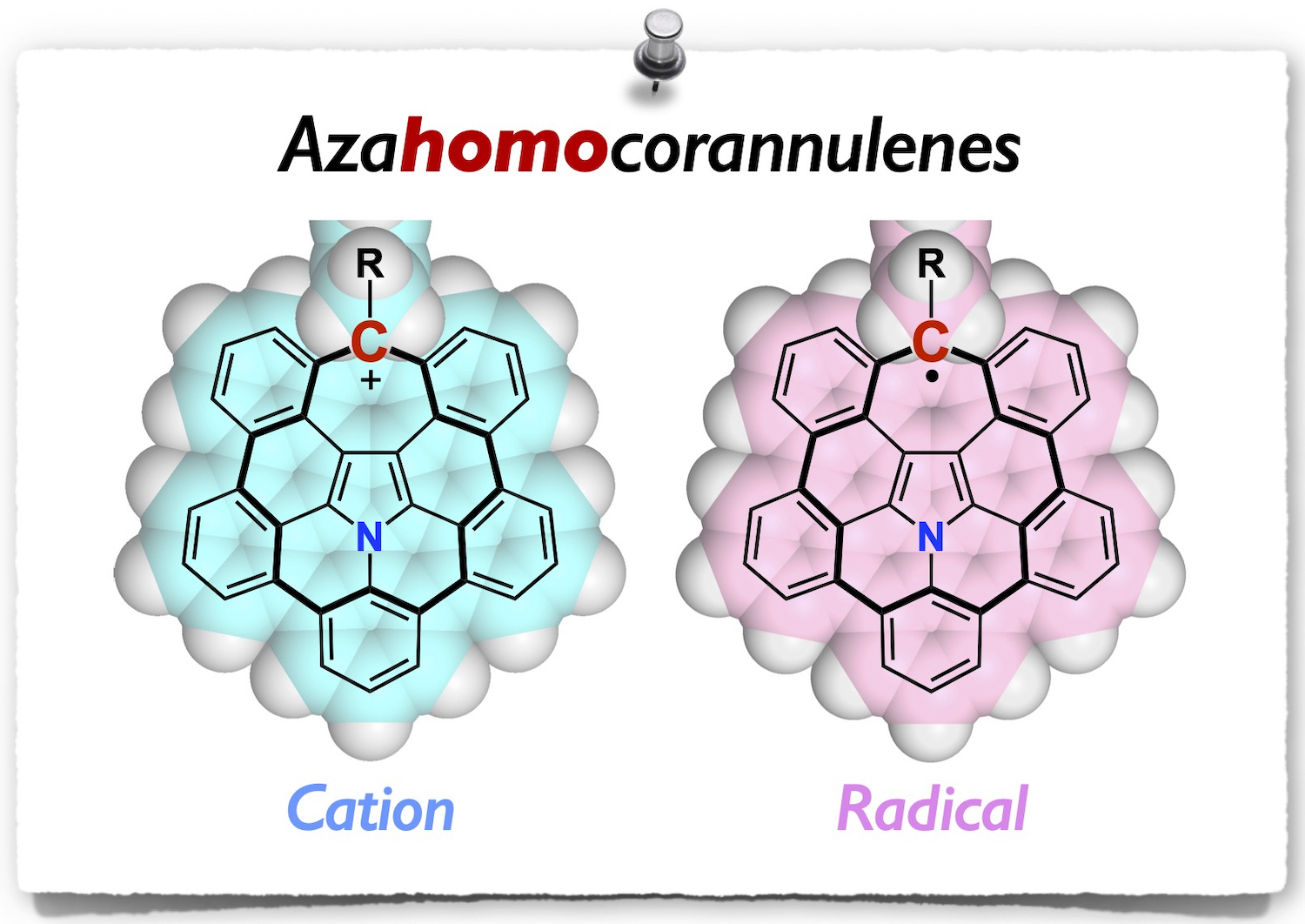
Abstract: Cycloheptatrienyl (tropyl) molecules are representative nonalternant hydrocarbons that offer interesting chemistry due to their unique structures and properties. However, there have been a limited number of polycyclic aromatic tropyl cations and radicals reported in the literature. Herein, we report the synthesis of a series of azahomocorannulene derivatives, where the key reactions are 1,3-dipolar cycloaddition of polycyclic aromatic azomethine ylides with dibenzotropone and a subsequent palladium-catalyzed cyclization. X-ray diffraction analysis revealed that the obtained azahomocorannulenyl cation and radical adopt planar structures and exhibit unique packing structures. Their electronic and optical properties were investigated experimentally and theoretically to reveal their aromatic character.