Z. Zhou, Z. Wei, Y. Tokimaru, S. Ito,* K. Nozaki,* M. A. Petrukhina*
Angew. Chem. Int. Ed. 2019, 58, 12107-12111. DOI: 10.1002/anie.201906748 and 10.1002/ange.201906748
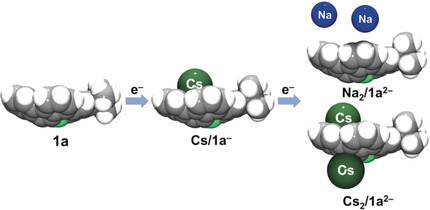
Abstract: Mono- and dianions of 2-tert-butyl-3a2-azapentabenzo[bc,ef,hi,kl,no]corannulene (1a) were synthesized by chemical reduction with sodium and cesium metals and crystallized as the corresponding salts in the presence of 18-crown-6 ether. X-ray diffraction analysis of the sodium salt, [{Na+(18-crown-6)(THF)2}3{Na+(18-crown-6)(THF)}(1a2–)2], revealed the presence of a naked dianion. In contrast, the controlled reactions of 1a with Cs allowed the isolation of mono- and doubly-reduced forms of 1a, both forming π-complexes with cesium ions in the solid state. In [{Cs+(18-crown-6)}(1a−)]∙THF, the asymmetric binding of the Cs+ ion to the concave surface of 1a− is observed, whereas in [{Cs+(18-crown-6)}2(1a2–)], two Cs+ ions bind to both concave and convex surfaces of the dianion. The present study provides the first successful isolation and characterization of the reduced products of heteroatom-containing buckybowl molecules.
