S. Ito, T. Itoh, M. Nakamura*
Angew. Chem. Int. Ed. 2011, 49, 454–457. DOI: 10.1002/anie.201006180
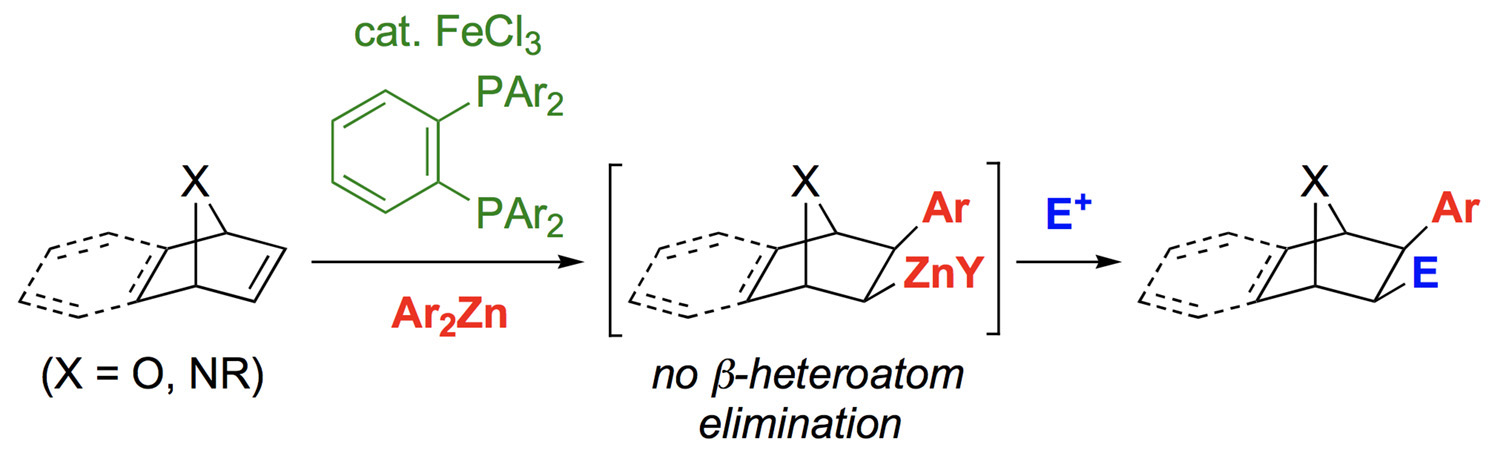
Abstract: Highly diastereoselective carbometalation of oxa- and azabicyclic alkenes with arylzinc reagents has been achieved by using FeCl3 and novel ortho-phenylene diphosphine ligands (see scheme; E=electrophile). The carbozincation products are stable towards β-heteroatom elimination and can be trapped with various electrophiles.